Protein found in humans
| TLR3 |
|---|
 |
| Available structures |
|---|
| PDB | Ortholog search: PDBe RCSB |
|---|
| List of PDB id codes |
|---|
1ZIW, 2A0Z, 3ULU, 3ULV, 2MK9, 2MKA |
|
|
| Identifiers |
|---|
| Aliases | TLR3, CD283, IIAE2, toll like receptor 3, IMD83 |
|---|
| External IDs | OMIM: 603029; MGI: 2156367; HomoloGene: 20696; GeneCards: TLR3; OMA:TLR3 - orthologs |
|---|
| Gene location (Human) |
|---|
 | | Chr. | Chromosome 4 (human)[1] |
|---|
| | Band | 4q35.1 | Start | 186,069,155 bp[1] |
|---|
| End | 186,088,073 bp[1] |
|---|
|
| Gene location (Mouse) |
|---|
 | | Chr. | Chromosome 8 (mouse)[2] |
|---|
| | Band | 8|8 B1.1 | Start | 45,848,702 bp[2] |
|---|
| End | 45,864,117 bp[2] |
|---|
|
| RNA expression pattern |
|---|
| Bgee | | Human | Mouse (ortholog) |
|---|
| Top expressed in | - jejunal mucosa
- palpebral conjunctiva
- placenta
- rectum
- sperm
- duodenum
- gallbladder
- Achilles tendon
- epithelium of colon
- pancreatic epithelial cell
|
| | Top expressed in | - conjunctival fornix
- gastrula
- stria vascularis
- transitional epithelium of urinary bladder
- vestibular membrane of cochlear duct
- vestibular sensory epithelium
- secondary oocyte
- lumbar subsegment of spinal cord
- Epithelium of choroid plexus
- jejunum
|
| | More reference expression data |
|
|---|
| BioGPS |  | | More reference expression data |
|
|---|
|
| Gene ontology |
|---|
| Molecular function | - protein binding
- identical protein binding
- RNA binding
- double-stranded RNA binding
- transmembrane signaling receptor activity
- signaling receptor activity
| | Cellular component | - cytoplasm
- integral component of membrane
- endosome
- endoplasmic reticulum membrane
- membrane
- Golgi membrane
- integral component of plasma membrane
- intracellular anatomical structure
- cell surface
- lysosomal membrane
- early endosome
- endoplasmic reticulum
- endolysosome membrane
- endosome membrane
- extracellular space
- extracellular matrix
| | Biological process | - positive regulation of interferon-beta production
- response to dsRNA
- defense response
- positive regulation of toll-like receptor signaling pathway
- positive regulation of protein phosphorylation
- male gonad development
- positive regulation of inflammatory response
- positive regulation of interleukin-12 production
- positive regulation of type III interferon production
- cellular response to interferon-beta
- response to exogenous dsRNA
- negative regulation of osteoclast differentiation
- positive regulation of cytokine production
- immune system process
- response to virus
- activation of NF-kappaB-inducing kinase activity
- I-kappaB phosphorylation
- positive regulation of JNK cascade
- hyperosmotic response
- cellular response to dsRNA
- regulation of dendritic cell cytokine production
- microglial cell activation
- toll-like receptor signaling pathway
- TRIF-dependent toll-like receptor signaling pathway
- cellular response to mechanical stimulus
- microglial cell activation involved in immune response
- positive regulation of gene expression
- defense response to bacterium
- positive regulation of NF-kappaB transcription factor activity
- defense response to virus
- necroptotic signaling pathway
- positive regulation of interleukin-8 production
- cellular response to exogenous dsRNA
- positive regulation of interleukin-6 production
- positive regulation of tumor necrosis factor production
- cellular response to interferon-gamma
- positive regulation of chemokine production
- positive regulation of apoptotic process
- positive regulation of I-kappaB kinase/NF-kappaB signaling
- detection of virus
- inflammatory response
- innate immune response
- I-kappaB kinase/NF-kappaB signaling
- toll-like receptor 3 signaling pathway
- positive regulation of type I interferon production
- MyD88-independent toll-like receptor signaling pathway
- signal transduction
- positive regulation of transcription by RNA polymerase II
- necroptosis
- apoptotic signaling pathway
- negative regulation of MyD88-independent toll-like receptor signaling pathway
- extrinsic apoptotic signaling pathway
- positive regulation of angiogenesis
- positive regulation of NIK/NF-kappaB signaling
| | Sources:Amigo / QuickGO |
|
| Orthologs |
|---|
| Species | Human | Mouse |
|---|
| Entrez | | |
|---|
| Ensembl | | |
|---|
| UniProt | | |
|---|
| RefSeq (mRNA) | | |
|---|
NM_126166
NM_001357316
NM_001357317 |
|
|---|
| RefSeq (protein) | | |
|---|
NP_569054
NP_001344245
NP_001344246 |
|
|---|
| Location (UCSC) | Chr 4: 186.07 – 186.09 Mb | Chr 8: 45.85 – 45.86 Mb |
|---|
| PubMed search | [3] | [4] |
|---|
|
| Wikidata |
| View/Edit Human | View/Edit Mouse |
|
Toll-like receptor 3 (TLR3) also known as CD283 (cluster of differentiation 283) is a protein that in humans is encoded by the TLR3 gene.[5] TLR3 is a member of the toll-like receptor family of pattern recognition receptors of the innate immune system. TLR3 recognizes double-stranded RNA in endosomes, which is a common feature of viral genomes internalised by macrophages and dendritic cells.
Function
TLR3 is a member of the toll-like receptor (TLR) family which plays a fundamental role in pathogen recognition and activation of innate immunity. TLRs are highly conserved from Drosophila to humans and share structural and functional similarities. They recognize pathogen-associated molecular patterns (PAMPs) that are expressed on infectious agents, and mediate the production of cytokines necessary for the development of effective immunity. The various TLRs exhibit different patterns of expression. This receptor is most abundantly expressed in placenta and pancreas, and is restricted to the dendritic subpopulation of the leukocytes. It recognizes dsRNA associated with viral infection, and induces the activation of IRF3 and NF-κB.[6] Unlike other TLRs, TLR3 uses TRIF as the sole adaptor.[6] IRF3 ultimately induces the production of type I interferons. It may thus play a role in host defense against viruses.[7]
TLR3 recognizes double-stranded RNA, a form of genetic information carried by some viruses such as reoviruses. Additionally, an ephemeral form of double-stranded RNA exists as a replicative intermediate during virus replication.[8] Upon recognition, TLR3 induces the activation of IRF3 to increase production of type I interferons which signal other cells to increase their antiviral defenses. Double-stranded RNA is also recognised by the cytoplasmic receptors RIG-I and MDA-5.[9]
TLR3 displays a protective role in mouse models of atherosclerosis,[10] and activation of TLR3 signaling is associated with ischemic preconditioning-induced protection against brain ischemia and attenuation of reactive astrogliosis.[11][12] Furthermore, TLR3 activation has been shown to promote hair follicle regeneration in skin wound healing.[13] In addition, TLR3 activators show effects on human vascular cells.[10]
Structure
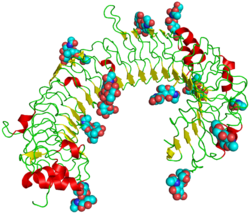
The structure of TLR3 was reported in June 2005 by researchers at The Scripps Research Institute.[14] TLR3 forms a large horseshoe shape that contacts with a neighboring horseshoe, forming a "dimer" of two horseshoes. Much of the TLR3 protein surface is covered with sugar molecules, making it a glycoprotein, but on one face (including the proposed interface between the two horseshoes), there is a large sugar-free surface. This surface also contains two distinct patches rich in positively charged amino acids, which may be a binding site for negatively charged double-stranded RNA.
Despite being a glycoprotein, TLR3 crystallises readily – a prerequisite for structural analysis by x-ray crystallography.
Ligands
- Antagonists
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000164342 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000031639 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF (January 1998). "A family of human receptors structurally related to Drosophila Toll". Proceedings of the National Academy of Sciences of the United States of America. 95 (2): 588–93. Bibcode:1998PNAS...95..588R. doi:10.1073/pnas.95.2.588. PMC 18464. PMID 9435236.
- ^ a b Kawai T, Akira S (November 2007). "Signaling to NF-kappaB by Toll-like receptors". Trends in Molecular Medicine. 13 (11): 460–9. doi:10.1016/j.molmed.2007.09.002. PMID 18029230.
- ^ "Entrez Gene: toll-like receptor 3".
- ^ Norval M (2012). "Virus–Cell Interactions". In Greenwood D, Slack RC, Barer MR, et al. (eds.). Medical Microbiology (18th ed.). Edinburgh: Churchill Livingstone. p. 88. ISBN 978-0-7020-4089-4.
- ^ Alexopoulou L, Holt AC, Medzhitov R, Flavell RA (October 2001). "Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3". Nature. 413 (6857): 732–8. Bibcode:2001Natur.413..732A. doi:10.1038/35099560. PMID 11607032. S2CID 4346537.
- ^ a b Cole JE, Navin TJ, Cross AJ, Goddard ME, Alexopoulou L, Mitra AT, Davies AH, Flavell RA, Feldmann M, Monaco C (February 2011). "Unexpected protective role for Toll-like receptor 3 in the arterial wall". Proceedings of the National Academy of Sciences of the United States of America. 108 (6): 2372–7. doi:10.1073/pnas.1018515108. PMC 3038746. PMID 21220319.
- ^ Pan LN, Zhu W, Li Y, Xu XL, Guo LJ, Lu Q, Wang J (2014). "Astrocytic Toll-like receptor 3 is associated with ischemic preconditioning-induced protection against brain ischemia in rodents". PLOS ONE. 9 (6): e99526. Bibcode:2014PLoSO...999526P. doi:10.1371/journal.pone.0099526. PMC 4051824. PMID 24914679.
- ^ Li Y, Xu XL, Zhao D, Pan LN, Huang CW, Guo LJ, Lu Q, Wang J (November 2015). "TLR3 ligand Poly IC Attenuates Reactive Astrogliosis and Improves Recovery of Rats after Focal Cerebral Ischemia". CNS Neuroscience & Therapeutics. 21 (11): 905–13. doi:10.1111/cns.12469. PMC 4638223. PMID 26494128.
- ^ Nelson AM, Reddy SK, Ratliff TS, Hossain MZ, Katseff AS, Zhu AS, Chang E, Resnik SR, Page C, Kim D, Whittam AJ, Miller LS, Garza LA (August 2015). "dsRNA Released by Tissue Damage Activates TLR3 to Drive Skin Regeneration". Cell Stem Cell. 17 (2): 139–51. doi:10.1016/j.stem.2015.07.008. PMC 4529957. PMID 26253200.
- ^ Choe J, Kelker MS, Wilson IA (July 2005). "Crystal structure of human toll-like receptor 3 (TLR3) ectodomain". Science. 309 (5734): 581–5. Bibcode:2005Sci...309..581C. doi:10.1126/science.1115253. PMID 15961631. S2CID 4962727.
Further reading
- Lien E, Ingalls RR (January 2002). "Toll-like receptors". Critical Care Medicine. 30 (1 Suppl): S1-11. doi:10.1097/00003246-200201001-00001. PMID 11782555.
- Wang PF, Fang H, Chen J, Lin S, Liu Y, Xiong XY, Wang YC, Xiong RP, Lv FL, Wang J, Yang QW (May 2014). "Polyinosinic-polycytidylic acid has therapeutic effects against cerebral ischemia/reperfusion injury through the downregulation of TLR4 signaling via TLR3". Journal of Immunology. 192 (10): 4783–94. doi:10.4049/jimmunol.1303108. PMC 4009499. PMID 24729619.
- Li Y, Xu XL, Zhao D, Pan LN, Huang CW, Guo LJ, Lu Q, Wang J (November 2015). "TLR3 ligand Poly IC Attenuates Reactive Astrogliosis and Improves Recovery of Rats after Focal Cerebral Ischemia". CNS Neuroscience & Therapeutics. 21 (11): 905–13. doi:10.1111/cns.12469. PMC 4638223. PMID 26494128.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.
|
|---|
| 1–50 | |
|---|
| 51–100 | |
|---|
| 101–150 | |
|---|
| 151–200 | |
|---|
| 201–250 | |
|---|
| 251–300 | |
|---|
| 301–350 | |
|---|

 1ziw: Human Toll-like Receptor 3 extracellular domain structure
1ziw: Human Toll-like Receptor 3 extracellular domain structure 2a0z: The molecular structure of toll-like receptor 3 ligand binding domain
2a0z: The molecular structure of toll-like receptor 3 ligand binding domain



















