PRKD3
| PRKD3 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | PRKD3, EPK2, PKC-NU, PKD3, PRKCN, nPKC-NU, protein kinase D3 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 607077; MGI: 1922542; HomoloGene: 2055; GeneCards: PRKD3; OMA:PRKD3 - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Serine/threonine-protein kinase D3 (PKD3) or PKC-nu is an enzyme that in humans is encoded by the PRKD3 gene.[5][6]
Protein kinase C (PKC) is a family of serine- and threonine-specific protein kinases that can be activated by calcium and the second messenger diacylglycerol. PKC family members phosphorylate a wide variety of protein targets and are known to be involved in diverse cellular signaling pathways. PKC family members also serve as major receptors for phorbol esters, a class of tumor promoters. Each member of the PKC family has a specific expression profile and is believed to play a distinct role. The protein encoded by this gene is one of the PKC family members. This kinase can be activated rapidly by the agonists of G protein-coupled receptors. It resides in both cytoplasm and nucleus, and its nuclear accumulation is found to be dramatically enhanced in response to its activation. This kinase can also be activated after B-cell antigen receptor (BCR) engagement, which requires intact phospholipase C gamma and the involvement of other PKC family members.[6]
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000115825 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000024070 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Hayashi A, Seki N, Hattori A, Kozuma S, Saito T (Jun 1999). "PKCnu, a new member of the protein kinase C family, composes a fourth subfamily with PKCmu". Biochim Biophys Acta. 1450 (1): 99–106. doi:10.1016/S0167-4889(99)00040-3. PMID 10231560.
- ^ a b "Entrez Gene: PRKD3 protein kinase D3".
Further reading
- Ali A, Hoeflich KP, Woodgett JR (2002). "Glycogen synthase kinase-3: properties, functions, and regulation". Chem. Rev. 101 (8): 2527–40. doi:10.1021/cr000110o. PMID 11749387.
- Jakobovits A, Rosenthal A, Capon DJ (1990). "Trans-activation of HIV-1 LTR-directed gene expression by tat requires protein kinase C". EMBO J. 9 (4): 1165–70. doi:10.1002/j.1460-2075.1990.tb08223.x. PMC 551792. PMID 2182321.
- Schultz SJ, Nigg EA (1994). "Identification of 21 novel human protein kinases, including 3 members of a family related to the cell cycle regulator nimA of Aspergillus nidulans". Cell Growth Differ. 4 (10): 821–30. PMID 8274451.
- Conant K, Ma M, Nath A, Major EO (1996). "Extracellular human immunodeficiency virus type 1 Tat protein is associated with an increase in both NF-kappa B binding and protein kinase C activity in primary human astrocytes". J. Virol. 70 (3): 1384–9. doi:10.1128/JVI.70.3.1384-1389.1996. PMC 189957. PMID 8627654.
- Holmes AM (1996). "In vitro phosphorylation of human immunodeficiency virus type 1 Tat protein by protein kinase C: evidence for the phosphorylation of amino acid residue serine-46". Arch. Biochem. Biophys. 335 (1): 8–12. doi:10.1006/abbi.1996.0476. PMID 8914829.
- Borgatti P, Zauli G, Cantley LC, Capitani S (1998). "Extracellular HIV-1 Tat protein induces a rapid and selective activation of protein kinase C (PKC)-alpha, and -epsilon and -zeta isoforms in PC12 cells". Biochem. Biophys. Res. Commun. 242 (2): 332–7. doi:10.1006/bbrc.1997.7877. PMID 9446795.
- Zidovetzki R, Wang JL, Chen P, et al. (1998). "Human immunodeficiency virus Tat protein induces interleukin 6 mRNA expression in human brain endothelial cells via protein kinase C- and cAMP-dependent protein kinase pathways". AIDS Res. Hum. Retroviruses. 14 (10): 825–33. doi:10.1089/aid.1998.14.825. PMID 9671211.
- Mayne M, Holden CP, Nath A, Geiger JD (2000). "Release of calcium from inositol 1,4,5-trisphosphate receptor-regulated stores by HIV-1 Tat regulates TNF-alpha production in human macrophages". J. Immunol. 164 (12): 6538–42. doi:10.4049/jimmunol.164.12.6538. PMID 10843712.
- Fang X, Yu SX, Lu Y, et al. (2000). "Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A". Proc. Natl. Acad. Sci. U.S.A. 97 (22): 11960–5. Bibcode:2000PNAS...9711960F. doi:10.1073/pnas.220413597. PMC 17277. PMID 11035810.
- Badou A, Bennasser Y, Moreau M, et al. (2000). "Tat Protein of Human Immunodeficiency Virus Type 1 Induces Interleukin-10 in Human Peripheral Blood Monocytes: Implication of Protein Kinase C-Dependent Pathway". J. Virol. 74 (22): 10551–62. doi:10.1128/JVI.74.22.10551-10562.2000. PMC 110929. PMID 11044099.
- Park IW, Wang JF, Groopman JE (2001). "HIV-1 Tat promotes monocyte chemoattractant protein-1 secretion followed by transmigration of monocytes". Blood. 97 (2): 352–8. doi:10.1182/blood.V97.2.352. PMID 11154208.
- Bennasser Y, Yamina B, Contreras X, et al. (2002). "[HIV-1 Tat protein induces IL-10 production by human monocytes: implications of the PKC and calcium pathway]". J. Soc. Biol. 195 (3): 319–26. PMID 11833470.
- Fang X, Yu S, Tanyi JL, et al. (2002). "Convergence of Multiple Signaling Cascades at Glycogen Synthase Kinase 3: Edg Receptor-Mediated Phosphorylation and Inactivation by Lysophosphatidic Acid through a Protein Kinase C-Dependent Intracellular Pathway". Mol. Cell. Biol. 22 (7): 2099–110. doi:10.1128/MCB.22.7.2099-2110.2002. PMC 133668. PMID 11884598.
- Bennasser Y, Bahraoui E (2002). "HIV-1 Tat protein induces interleukin-10 in human peripheral blood monocytes: involvement of protein kinase C-betaII and -delta". FASEB J. 16 (6): 546–54. doi:10.1096/fj.01-0775com. PMID 11919157. S2CID 84545613.
- Dai F, Yu L, He H, et al. (2002). "Human serum and glucocorticoid-inducible kinase-like kinase (SGKL) phosphorylates glycogen syntheses kinase 3 beta (GSK-3beta) at serine-9 through direct interaction". Biochem. Biophys. Res. Commun. 293 (4): 1191–6. doi:10.1016/S0006-291X(02)00349-2. PMID 12054501.
- Efimova T, Deucher A, Kuroki T, et al. (2002). "Novel protein kinase C isoforms regulate human keratinocyte differentiation by activating a p38 delta mitogen-activated protein kinase cascade that targets CCAAT/enhancer-binding protein alpha". J. Biol. Chem. 277 (35): 31753–60. doi:10.1074/jbc.M205098200. PMID 12080077.
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. Bibcode:2002PNAS...9916899M. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Bennasser Y, Badou A, Tkaczuk J, Bahraoui E (2003). "Signaling pathways triggered by HIV-1 Tat in human monocytes to induce TNF-alpha". Virology. 303 (1): 174–80. doi:10.1006/viro.2002.1676. PMID 12482669.
- v
- t
- e
-
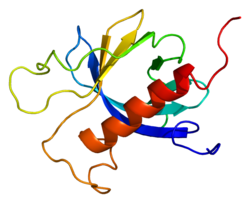
 2d9z: Solution structure of the PH domain of Protein kinase C, nu type from human
2d9z: Solution structure of the PH domain of Protein kinase C, nu type from human
 | This article on a gene on human chromosome 2 is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e