Rhodionin
 | |
| Names | |
|---|---|
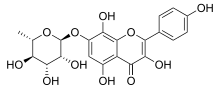
| IUPAC name 3,5,8-Trihydroxy-2-(4-hydroxyphenyl)-7-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxychromen-4-one | |
| Other names Herbacetin-7-O-α-L-rhamnopyranoside Herbacetin 7-rhamnoside | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
Chemical formula | C21H20O11 |
| Molar mass | 448.380 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). Infobox references | |
Chemical compound
Rhodionin is a herbacetin rhamnoside found in Rhodiola species.[1]
References
- ^ Li, T.; Zhang, H. (2008). "Identification and Comparative Determination of Rhodionin in Traditional Tibetan Medicinal Plants of Fourteen Rhodiola Species by High-Performance Liquid Chromatography-Photodiode Array Detection and Electrospray Ionization-Mass Spectrometry". Chemical & Pharmaceutical Bulletin. 56 (6): 807–14. doi:10.1248/cpb.56.807. PMID 18520085.
- v
- t
- e
Flavonols and their conjugates
| Aglycones |
|
|---|
| Aglycones |
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conjugates |
|
| Aglycones |
| ||||
|---|---|---|---|---|---|
| Glycosides |
|
| Aglycones |
|
|---|---|
| Glycosides |
|
| Aglycones |
|---|
| Aglycones | |
|---|---|
| Glycosides |
|
| Glycosides |
|---|
 | This article about an aromatic compound is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e













