Kaempferitrin
 | |
| Names | |
|---|---|
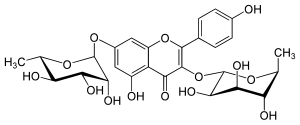
| IUPAC name 4′,5-Dihydroxy-3,7-bis(α-L-rhamnopyranosyloxy)flavone | |
| Systematic IUPAC name 5-Hydroxy-2-(4-hydroxyphenyl)-3,7-bis{[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy}-4H-1-benzopyran-4-one | |
| Other names Lespenefril Lespenephryl Lespedin lespenephril Kaempferol 3,7-dirhamnoside Kaempferol 3,7 bisrhamnoside | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI |
|
| ChEMBL |
|
| ChemSpider |
|
| KEGG |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| Properties | |
Chemical formula | C27H30O14 |
| Molar mass | 578.52 g/mol |
| Density | 1.7 g/mL |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).  Y verify (what is Y verify (what is  Y Y N ?) N ?) Infobox references | |
Chemical compound
Kaempferitrin is a chemical compound. It can be isolated from the leaves of Hedyotis verticillata[1] and from Onychium japonicum.[2]
Kaempferitrin is the 3,7-dirhamnoside of kaempferol.
References
- ^ Hamzah, Ahmad Sazali; Lajis, Nordin H.; Sargent, M. V. (1994). "Kaempferitrin from the leaves of Hedyotis verticillata and its biological activity". Planta Medica. 60 (4): 388–389. doi:10.1055/s-2006-959513. PMID 7938277. S2CID 42952045. INIST 3324508.
- ^ Kobayashi, Kenji (1944). "Tachi shinobu no ichi seibun Kaempferol-birhamnosid ni shūte" [On kaempferol dirhamnoside, one component of carrot fern]. Yakugaku zasshi (in Japanese). 64 (9): 35. doi:10.1248/yakushi1944a.64.9_35a. S2CID 100133209.
- v
- t
- e
Flavonols and their conjugates
| Aglycones |
|
|---|
| Aglycones |
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conjugates |
|
| Aglycones |
| ||||
|---|---|---|---|---|---|
| Glycosides |
|
| Aglycones |
|
|---|---|
| Glycosides |
|
| Aglycones |
|---|
| Aglycones | |
|---|---|
| Glycosides |
|
| Glycosides |
|---|
 | This article about an aromatic compound is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e










