Cathepsin H
| CTSH | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
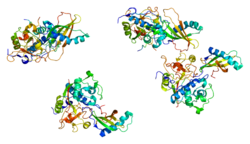 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | CTSH, ACC-4, ACC-5, CPSB, minichain, ACC4, ACC5, cathepsin H | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 116820; MGI: 107285; HomoloGene: 36159; GeneCards: CTSH; OMA:CTSH - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Cathepsin H is a protein that in humans is encoded by the CTSH gene.[5][6]
The protein encoded by this gene is a cysteine cathepsin, a lysosomal cysteine protease important in the overall degradation of lysosomal proteins. It is composed of a dimer of disulfide-linked heavy and light chains, both produced from a single protein precursor. The encoded protein, which belongs to the peptidase C1 protein family, can act both as an aminopeptidase and as an endopeptidase. Increased expression of this gene has been correlated with malignant progression of prostate tumors. Two transcript variants encoding different isoforms have been found for this gene.[6]
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000103811 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000032359 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Fuchs R, Machleidt W, Gassen HG (Feb 1989). "Molecular cloning and sequencing of a cDNA coding for mature human kidney cathepsin H". Biol Chem Hoppe-Seyler. 369 (6): 469–75. doi:10.1515/bchm3.1988.369.1.469. PMID 2849458.
- ^ a b "Entrez Gene: CTSH cathepsin H".
Further reading
- Sawicki G, Warwas M (1990). "Cathepsin H from human placenta". Acta Biochim. Pol. 36 (3–4): 343–51. PMID 2486008.
- Fuchs R, Gassen HG (1990). "Nucleotide sequence of human preprocathepsin H, a lysosomal cysteine proteinase". Nucleic Acids Res. 17 (22): 9471. doi:10.1093/nar/17.22.9471. PMC 335148. PMID 2587265.
- Chernaia VI, Reva AD (1990). "[Cathepsin H activity in the human brain and human brain neoplasms]". Ukr. Biokhim. Zh. 61 (5): 47–50. PMID 2588347.
- Ritonja A, Popović T, Kotnik M, et al. (1988). "Amino acid sequences of the human kidney cathepsins H and L." FEBS Lett. 228 (2): 341–5. doi:10.1016/0014-5793(88)80028-0. PMID 3342889.
- Järvinen M, Rinne A (1983). "Human spleen cysteineproteinase inhibitor. Purification, fractionation into isoelectric variants and some properties of the variants". Biochim. Biophys. Acta. 708 (2): 210–7. doi:10.1016/0167-4838(82)90222-9. PMID 6184075.
- Kato S, Sekine S, Oh SW, et al. (1995). "Construction of a human full-length cDNA bank". Gene. 150 (2): 243–50. doi:10.1016/0378-1119(94)90433-2. PMID 7821789.
- Baumgrass R, Williamson MK, Price PA (1997). "Identification of peptide fragments generated by digestion of bovine and human osteocalcin with the lysosomal proteinases cathepsin B, D, L, H, and S." J. Bone Miner. Res. 12 (3): 447–55. doi:10.1359/jbmr.1997.12.3.447. PMID 9076588.
- Söderström M, Salminen H, Glumoff V, et al. (1999). "Cathepsin expression during skeletal development". Biochim. Biophys. Acta. 1446 (1–2): 35–46. doi:10.1016/S0167-4781(99)00068-8. PMID 10395917.
- Jokimaa V, Oksjoki S, Kujari H, et al. (2001). "Expression patterns of cathepsins B, H, K, L and S in the human endometrium". Mol. Hum. Reprod. 7 (1): 73–8. doi:10.1093/molehr/7.1.73. PMID 11134363.
- Uusitalo H, Hiltunen A, Söderström M, et al. (2001). "Expression of cathepsins B, H, K, L, and S and matrix metalloproteinases 9 and 13 during chondrocyte hypertrophy and endochondral ossification in mouse fracture callus". Calcif. Tissue Int. 67 (5): 382–90. doi:10.1007/s002230001152. PMID 11136537. S2CID 31004810.
- Pol E, Björk I (2001). "Role of the single cysteine residue, Cys 3, of human and bovine cystatin B (stefin B) in the inhibition of cysteine proteinases". Protein Sci. 10 (9): 1729–38. doi:10.1110/ps.11901. PMC 2253190. PMID 11514663.
- Waghray A, Keppler D, Sloane BF, et al. (2002). "Analysis of a truncated form of cathepsin H in human prostate tumor cells". J. Biol. Chem. 277 (13): 11533–8. doi:10.1074/jbc.M109557200. PMID 11796715.
- Brasch F, Ten Brinke A, Johnen G, et al. (2002). "Involvement of cathepsin H in the processing of the hydrophobic surfactant-associated protein C in type II pneumocytes". Am. J. Respir. Cell Mol. Biol. 26 (6): 659–70. doi:10.1165/ajrcmb.26.6.4744. PMID 12034564.
- Bühling F, Waldburg N, Krüger S, et al. (2003). "Expression of cathepsins B, H, K, L, and S during human fetal lung development". Dev. Dyn. 225 (1): 14–21. doi:10.1002/dvdy.10134. PMID 12203716.
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. Bibcode:2002PNAS...9916899M. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Jenko S, Dolenc I, Guncar G, et al. (2003). "Crystal structure of Stefin A in complex with cathepsin H: N-terminal residues of inhibitors can adapt to the active sites of endo- and exopeptidases". J. Mol. Biol. 326 (3): 875–85. doi:10.1016/S0022-2836(02)01432-8. PMID 12581647.
- Nagai A, Terashima M, Harada T, et al. (2003). "Cathepsin B and H activities and cystatin C concentrations in cerebrospinal fluid from patients with leptomeningeal metastasis". Clin. Chim. Acta. 329 (1–2): 53–60. doi:10.1016/S0009-8981(03)00023-8. PMID 12589965.
- Han SR, Momeni A, Strach K, et al. (2004). "Enzymatically modified LDL induces cathepsin H in human monocytes: potential relevance in early atherogenesis". Arterioscler. Thromb. Vasc. Biol. 23 (4): 661–7. doi:10.1161/01.ATV.0000063614.21233.BF. PMID 12615673.
- Dodt J, Reichwein J (2004). "Human cathepsin H: deletion of the mini-chain switches substrate specificity from aminopeptidase to endopeptidase". Biol. Chem. 384 (9): 1327–32. doi:10.1515/BC.2003.149. PMID 14515996. S2CID 23726105.
External links
- The MEROPS online database for peptidases and their inhibitors: C01.040
- v
- t
- e
-
 1nb3: Crystal structure of stefin A in complex with cathepsin H: N-terminal residues of inhibitors can adapt to the active sites of endo-and exopeptidases
1nb3: Crystal structure of stefin A in complex with cathepsin H: N-terminal residues of inhibitors can adapt to the active sites of endo-and exopeptidases -
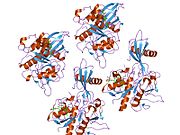 1nb5: Crystal structure of stefin A in complex with cathepsin H
1nb5: Crystal structure of stefin A in complex with cathepsin H -
 8pch: CRYSTAL STRUCTURE OF PORCINE CATHEPSIN H DETERMINED AT 2.1 ANGSTROM RESOLUTION: LOCATION OF THE MINI-CHAIN C-TERMINAL CARBOXYL GROUP DEFINES CATHEPSIN H AMINOPEPTIDASE FUNCTION
8pch: CRYSTAL STRUCTURE OF PORCINE CATHEPSIN H DETERMINED AT 2.1 ANGSTROM RESOLUTION: LOCATION OF THE MINI-CHAIN C-TERMINAL CARBOXYL GROUP DEFINES CATHEPSIN H AMINOPEPTIDASE FUNCTION
 | This article on a gene on human chromosome 15 is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e