Protein-coding gene in the species Homo sapiens
| CAPN2 |
|---|
 |
| Available structures |
|---|
| PDB | Ortholog search: PDBe RCSB |
|---|
| List of PDB id codes |
|---|
1KFU, 1KFX, 2NQA |
|
|
| Identifiers |
|---|
| Aliases | CAPN2, CANP2, CANPL2, CANPml, mCANP, calpain 2 |
|---|
| External IDs | OMIM: 114230; MGI: 88264; HomoloGene: 1326; GeneCards: CAPN2; OMA:CAPN2 - orthologs |
|---|
| Gene location (Human) |
|---|
 | | Chr. | Chromosome 1 (human)[1] |
|---|
| | Band | 1q41 | Start | 223,701,593 bp[1] |
|---|
| End | 223,776,018 bp[1] |
|---|
|
| Gene location (Mouse) |
|---|
 | | Chr. | Chromosome 1 (mouse)[2] |
|---|
| | Band | 1|1 H5 | Start | 182,294,825 bp[2] |
|---|
| End | 182,345,173 bp[2] |
|---|
|
| RNA expression pattern |
|---|
| Bgee | | Human | Mouse (ortholog) |
|---|
| Top expressed in | - bronchial epithelial cell
- nasal epithelium
- glomerulus
- mucosa of sigmoid colon
- right uterine tube
- parotid gland
- metanephric glomerulus
- parietal pleura
- cartilage tissue
- visceral pleura
|
| | Top expressed in | - endothelial cell of lymphatic vessel
- genital tubercle
- facial motor nucleus
- gastrula
- belly cord
- left lung
- tail of embryo
- right lung
- right lung lobe
- cornea
|
| | More reference expression data |
|
|---|
| BioGPS | 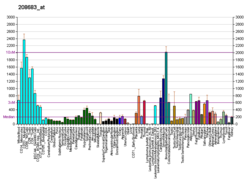 | | More reference expression data |
|
|---|
|
| Gene ontology |
|---|
| Molecular function | - calcium ion binding
- cysteine-type peptidase activity
- metal ion binding
- cytoskeletal protein binding
- peptidase activity
- protein binding
- protein heterodimerization activity
- hydrolase activity
- calcium-dependent cysteine-type endopeptidase activity
- enzyme binding
| | Cellular component | - Golgi apparatus
- pseudopodium
- membrane
- focal adhesion
- perinuclear endoplasmic reticulum
- intracellular anatomical structure
- dendrite
- endoplasmic reticulum
- cortical actin cytoskeleton
- membrane raft
- chromatin
- lysosome
- extracellular exosome
- nucleus
- cytosol
- cytoplasm
- plasma membrane
- mitochondrial intermembrane space
- external side of plasma membrane
- cell projection
- neuronal cell body
| | Biological process | - response to hypoxia
- protein autoprocessing
- proteolysis
- cellular response to amino acid stimulus
- myoblast fusion
- proteolysis involved in cellular protein catabolic process
- regulation of cytoskeleton organization
- blastocyst development
- extracellular matrix disassembly
- female pregnancy
- positive regulation of cardiac muscle cell apoptotic process
- regulation of interleukin-6 production
- cellular response to interferon-beta
- response to hydrogen peroxide
- behavioral response to pain
- cellular response to lipopolysaccharide
- positive regulation of neuron death
- positive regulation of myoblast fusion
- positive regulation of phosphatidylcholine biosynthetic process
| | Sources:Amigo / QuickGO |
|
|
| Wikidata |
| View/Edit Human | View/Edit Mouse |
|
Calpain-2 catalytic subunit is a protein that in humans is encoded by the CAPN2 gene.[5][6]
Function
The calpains, calcium-activated neutral proteases, are nonlysosomal, intracellular cysteine proteases. The mammalian calpains include ubiquitous, stomach-specific, and muscle-specific proteins. The ubiquitous enzymes consist of heterodimers with distinct large, catalytic subunits associated with a common small, regulatory subunit. This gene encodes the large subunit of the ubiquitous enzyme, calpain 2. Multiple heterogeneous transcriptional start sites in the 5' UTR have been reported.[7]
Interactions
CAPN2 has been shown to interact with Bcl-2.[8]
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000162909 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000026509 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Imajoh S, Aoki K, Ohno S, Emori Y, Kawasaki H, Sugihara H, Suzuki K (1988). "Molecular cloning of the cDNA for the large subunit of the high-Ca2+-requiring form of human Ca2+-activated neutral protease". Biochemistry. 27 (21): 8122–8. doi:10.1021/bi00421a022. PMID 2852952.
- ^ Hata A, Ohno S, Akita Y, Suzuki K (May 1989). "Tandemly reiterated negative enhancer-like elements regulate transcription of a human gene for the large subunit of calcium-dependent protease". J. Biol. Chem. 264 (11): 6404–11. doi:10.1016/S0021-9258(18)83364-6. PMID 2539381.
- ^ "Entrez Gene: CAPN2 calpain 2, (m/II) large subunit".
- ^ Gil-Parrado S, Fernández-Montalván A, Assfalg-Machleidt I, Popp O, Bestvater F, Holloschi A, Knoch TA, Auerswald EA, Welsh K, Reed JC, Fritz H, Fuentes-Prior P, Spiess E, Salvesen GS, Machleidt W (Jul 2002). "Ionomycin-activated calpain triggers apoptosis. A probable role for Bcl-2 family members". J. Biol. Chem. 277 (30): 27217–26. doi:10.1074/jbc.M202945200. PMID 12000759.
Further reading
- Suzuki K, Sorimachi H, Yoshizawa T, Kinbara K, Ishiura S (1995). "Calpain: novel family members, activation, and physiologic function". Biol. Chem. Hoppe-Seyler. 376 (9): 523–9. doi:10.1515/bchm3.1995.376.9.523. PMID 8561910.
- Cohen GM (1997). "Caspases: the executioners of apoptosis". Biochem. J. 326 (Pt 1): 1–16. doi:10.1042/bj3260001. PMC 1218630. PMID 9337844.
- Reverter D, Sorimachi H, Bode W (2001). "The structure of calcium-free human m-calpain: implications for calcium activation and function". Trends Cardiovasc. Med. 11 (6): 222–9. doi:10.1016/S1050-1738(01)00112-8. PMID 11673052.
- Kopp S (1976). "Reproducibility of response to a questionnaire on symptoms of masticatory dysfunction". Community Dent Oral Epidemiol. 4 (5): 205–9. doi:10.1111/j.1600-0528.1976.tb00985.x. PMID 1067155.
- Adachi Y, Kitahara-Ozawa A, Sugamura K, Lee WJ, Yodoi J, Maki M, Murachi T, Hatanaka M (1992). "Expression of calpain II gene in human hematopoietic system cells infected with human T-cell leukemia virus type I". J. Biol. Chem. 267 (27): 19373–8. doi:10.1016/S0021-9258(18)41785-1. PMID 1527057.
- Ohno S, Minoshima S, Kudoh J, Fukuyama R, Shimizu Y, Ohmi-Imajoh S, Shimizu N, Suzuki K (1990). "Four genes for the calpain family locate on four distinct human chromosomes". Cytogenet. Cell Genet. 53 (4): 225–9. doi:10.1159/000132937. PMID 2209092.
- Ishiguro H, Higashiyama S, Namikawa C, Kunimatsu M, Takano E, Tanaka K, Ohkubo I, Murachi T, Sasaki M (1987). "Interaction of human calpains I and II with high molecular weight and low molecular weight kininogens and their heavy chain: mechanism of interaction and the role of divalent cations". Biochemistry. 26 (10): 2863–70. doi:10.1021/bi00384a030. PMID 3038169.
- Srinivasula SM, Fernandes-Alnemri T, Zangrilli J, Robertson N, Armstrong RC, Wang L, Trapani JA, Tomaselli KJ, Litwack G, Alnemri ES (1996). "The Ced-3/interleukin 1beta converting enzyme-like homolog Mch6 and the lamin-cleaving enzyme Mch2alpha are substrates for the apoptotic mediator CPP32". J. Biol. Chem. 271 (43): 27099–106. doi:10.1074/jbc.271.43.27099. PMID 8900201.
- Corasaniti MT, Navarra M, Catani MV, Melino G, Nisticò G, Finazzi-Agrò A (1996). "NMDA and HIV-1 coat protein, GP120, produce necrotic but not apoptotic cell death in human CHP100 neuroblastoma cultures via a mechanism involving calpain". Biochem. Biophys. Res. Commun. 229 (1): 299–304. doi:10.1006/bbrc.1996.1796. PMID 8954122.
- Fujitani K, Kambayashi J, Sakon M, Ohmi SI, Kawashima S, Yukawa M, Yano Y, Miyoshi H, Ikeda M, Shinoki N, Monden M (1997). "Identification of mu-, m-calpains and calpastatin and capture of mu-calpain activation in endothelial cells". J. Cell. Biochem. 66 (2): 197–209. doi:10.1002/(SICI)1097-4644(19970801)66:2<197::AID-JCB7>3.0.CO;2-L. PMID 9213221. S2CID 85163404.
- Rock MT, Brooks WH, Roszman TL (1997). "Calcium-dependent signaling pathways in T cells. Potential role of calpain, protein tyrosine phosphatase 1b, and p130Cas in integrin-mediated signaling events". J. Biol. Chem. 272 (52): 33377–83. doi:10.1074/jbc.272.52.33377. PMID 9407132.
- Ueyama H, Kumamoto T, Fujimoto S, Murakami T, Tsuda T (1998). "Expression of three calpain isoform genes in human skeletal muscles". J. Neurol. Sci. 155 (2): 163–9. doi:10.1016/S0022-510X(97)00309-2. PMID 9562261. S2CID 205898607.
- Strobl S, Fernandez-Catalan C, Braun M, Huber R, Masumoto H, Nakagawa K, Irie A, Sorimachi H, Bourenkow G, Bartunik H, Suzuki K, Bode W (2000). "The crystal structure of calcium-free human m-calpain suggests an electrostatic switch mechanism for activation by calcium". Proc. Natl. Acad. Sci. U.S.A. 97 (2): 588–92. Bibcode:2000PNAS...97..588S. doi:10.1073/pnas.97.2.588. PMC 15374. PMID 10639123.
- Masumoto H, Nakagawa K, Irie S, Sorimachi H, Suzuki K, Bourenkov GP, Bartunik H, Fernandez-Catalan C, Bode W, Strobl S (2000). "Crystallization and preliminary X-ray analysis of recombinant full-length human m-calpain". Acta Crystallogr. D. 56 (Pt 1): 73–5. Bibcode:2000AcCrD..56...73M. doi:10.1107/S0907444999013748. PMID 10666632.
- Chua BT, Guo K, Li P (2000). "Direct cleavage by the calcium-activated protease calpain can lead to inactivation of caspases". J. Biol. Chem. 275 (7): 5131–5. doi:10.1074/jbc.275.7.5131. PMID 10671558.
- Lee MS, Kwon YT, Li M, Peng J, Friedlander RM, Tsai LH (2000). "Neurotoxicity induces cleavage of p35 to p25 by calpain". Nature. 405 (6784): 360–4. Bibcode:2000Natur.405..360L. doi:10.1038/35012636. PMID 10830966. S2CID 205006589.
External links
PDB gallery
-
1df0: CRYSTAL STRUCTURE OF M-CALPAIN -
1kfu: Crystal Structure of Human m-Calpain Form II -
1kfx: Crystal Structure of Human m-Calpain Form I -
1mdw: Crystal Structure of Calcium-Bound Protease Core of Calpain II Reveals the Basis for Intrinsic Inactivation -
1u5i: Crystal Structure analysis of rat m-calpain mutant Lys10 Thr |
 | This article on a gene on human chromosome 1 is a stub. You can help Wikipedia by expanding it. |

 1df0: CRYSTAL STRUCTURE OF M-CALPAIN
1df0: CRYSTAL STRUCTURE OF M-CALPAIN 1kfu: Crystal Structure of Human m-Calpain Form II
1kfu: Crystal Structure of Human m-Calpain Form II 1kfx: Crystal Structure of Human m-Calpain Form I
1kfx: Crystal Structure of Human m-Calpain Form I 1mdw: Crystal Structure of Calcium-Bound Protease Core of Calpain II Reveals the Basis for Intrinsic Inactivation
1mdw: Crystal Structure of Calcium-Bound Protease Core of Calpain II Reveals the Basis for Intrinsic Inactivation 1u5i: Crystal Structure analysis of rat m-calpain mutant Lys10 Thr
1u5i: Crystal Structure analysis of rat m-calpain mutant Lys10 Thr






















